

The discovery of the periodic system for classifying the elements represents the culmination of a number of scientific developments, rather than a sudden brainstorm on the part of one individual. (In the modern periodic table, a group or family corresponds to one vertical column.) Fortunately, the periodic table allows chemists to function by mastering the properties of a handful of typical elements all the others fall into so-called groups or families with similar chemical properties. Were it not for the simplification provided by this chart, students of chemistry would need to learn the properties of all 112 known elements.
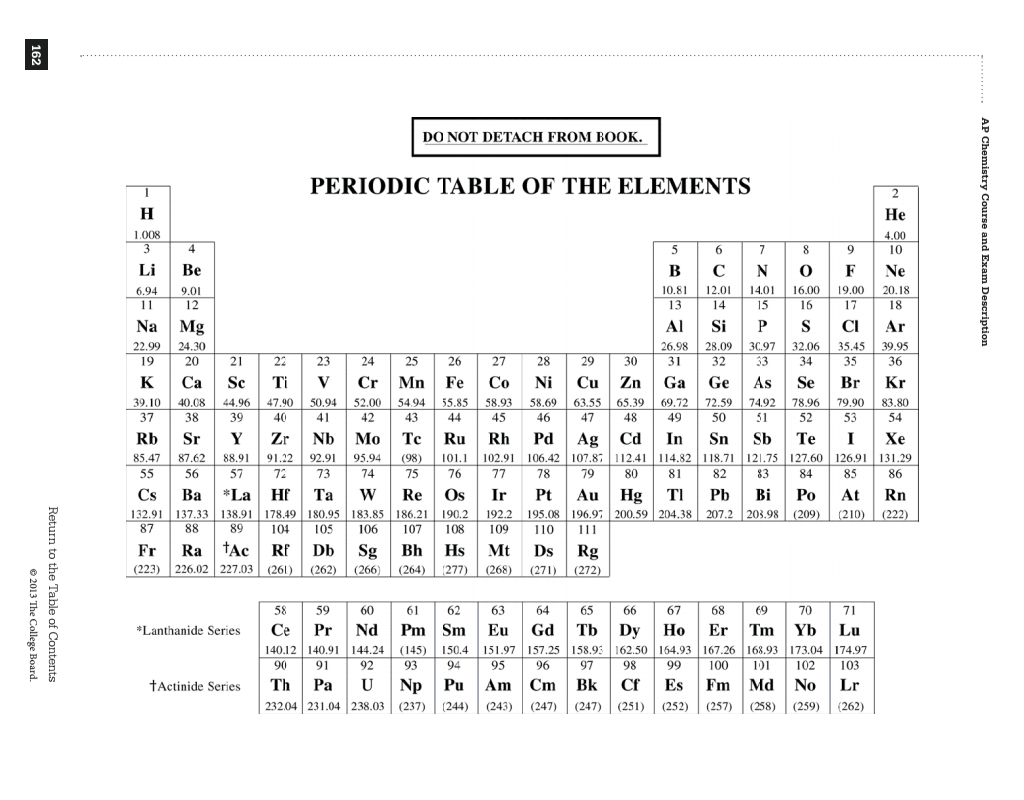
The term “periodic” reflects the fact that the elements show patterns in their chemical properties in certain regular intervals. Remarkably, the periodic table is thus notable both for its historical roots and for its modern relevance. In some instances, new findings initially appeared to call into question the theoretical foundations of the periodic table, but each time scientists eventually managed to incorporate the results while preserving the table’s fundamental structure. But despite the dramatic changes that have taken place in science over the past century-namely, the development of the theories of relativity and quantum mechanics-there has been no revolution in the basic nature of the periodic system. Throughout its long history, the periodic table has been disputed, altered and improved as science has progressed and as new elements have been discovered. The story of the periodic system for classifying the elements can be traced back over 200 years. Indeed, nothing quite like it exists in the other disciplines of science. A version hangs on the wall of nearly every chemical laboratory and lecture hall in the world. The periodic table of the elements is one of the most powerful icons in science: a single document that consolidates much of our knowledge of chemistry. The complete version with artwork is available for purchase here (PDF). "synopsis" may belong to another edition of this title.Editor's note: The following is a text-only version. The same periodic table in large format covers the walls in the UCLA Court of Sciences lecture halls. Great for high-school, college, and university students. Fits into textbook or notebook as a useful bookmark. Terrific easy to use information-rich resource for quick reference to the chemical elements, with well-known constants and formulas used in Chemistry, Biochemistry, and Physics on the reverse side. On the reverse page reference to constants in different units, conversions, and useful equations in Chemistry, Biochemistry, and Physics makes it an all-in-one resource. Both students and professionals will find identification of the Alkali Metals, Alkaline Earth Metals, Coinage Metals, Chalcogens, Halogens, Noble Gases, Rare Earth Metals, Lanthanides, Actinides, and Metalloids highly useful. The s-block, p-block, d-block, and f-block elements are easy to locate. All Groups and Periods are shown using both IUPAC and CAS notation, along with highlighting of radioactive elements makes it highly informative. Color coded for easy classification of solids, liquids, and gases at room temperature. Visually appealing layout of Symbol, Name, Atomic Number, Atomic Weight, and Electron Configuration for each element. Excellent presentation of the Periodic Table.


 0 kommentar(er)
0 kommentar(er)
